
Past Regents Questions on Entropy Jan 201044 Given the balanced equation representing a phase change:
C6H4Cl2(s) + energy==>C6H4Cl2(g)
Which statement describes this change?
(1) It is endothermic, and entropy decreases.
(2) It is endothermic, and entropy increases.
(3) It is exothermic, and entropy decreases.
(4) It is exothermic, and entropy increases. Aug 2009-45 The entropy of a sample of H2O increases as the sample changes from a
(1) gas to a liquid (3) liquid to a gas
(2) gas to a solid (4) liquid to a solid June 2008-45 Which 1-mole sample has the least entropy?
(1) Br2(s) at 266 K (3) Br2(l) at 332 K
(2) Br2(l) at 266 K (4) Br2(g) at 332 K June 2008-17 A thermometer is in a beaker of water. Which statement best explains why the thermometer reading initially increases when LiBr(s) is dissolved in the water?
(1) The entropy of the LiBr(aq) is greater than the entropy of the water.
(2) The entropy of the LiBr(aq) is less than the entropy of the water.
(3) The dissolving of the LiBr(s) in water is an endothermic process.
(4) The dissolving of the LiBr(s) in water is an exothermic process. Jan 2007-27 In terms of energy and entropy, systems in nature tend to undergo changes toward
(1) higher energy and higher entropy
(2) higher energy and lower entropy
(3) lower energy and higher entropy
(4) lower energy and lower entropy Aug 2006-49 Which list of the phases of H2O is arranged in order of increasing entropy?
(1) ice, steam, and liquid water
(2) ice, liquid water, and steam
(3) steam, liquid water, and ice
(4) steam, ice, and liquid water Jan 2006-8 Given the balanced equation:
I2(s) + energy ==> I2(g)
As a sample of I2(s) sublimes to I2(g), the entropy of the sample
(1) increases because the particles are less randomly arranged
(2) increases because the particles are more randomly arranged
(3) decreases because the particles are less randomly arranged
(4) decreases because the particles are more randomly arranged Aug 2005-46 At STP, a sample of which element has the highest entropy?
(1) Na(s) (3) Br2(l)
(2) Hg(l) (4) F2(g) June 2005-20 Systems in nature tend to undergo changes toward
(1) lower energy and lower entropy
(2) lower energy and higher entropy
(3) higher energy and lower entropy
(4) higher energy and higher entropy Jan 2005-43 Which of these changes produces the greatest
increase in entropy?
(1) CaCO3(s) --> CaO(s) + CO2(g)
(2) 2 Mg(s) + O2(g) --> 2 MgO(s)
(3) H2O(g) --> H2O(l)
(4) CO2(g) --> CO2(s) Aug 2004-21 Even though the process is endothermic, snow can sublime. Which tendency in nature accounts for this phase change?(1) a tendency toward greater entropy (2) a tendency toward greater energy(3) a tendency toward less entropy(4) a tendency toward less energyJan 2004- 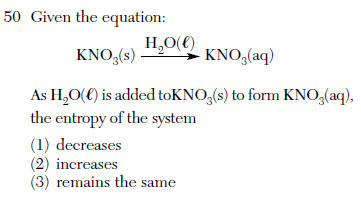
June 2005- 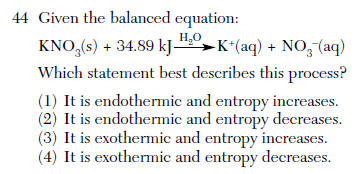
June 2008-22 Systems in nature tend to undergo changes toward
(1) lower energy and less disorder
(2) lower energy and more disorder
(3) higher energy and less disorder
(4) higher energy and more disorder June 2004- 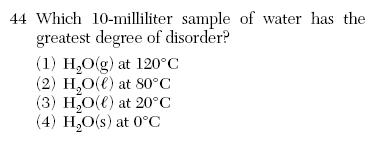
June 2003-50 As carbon dioxide sublimes, its entropy (1) decreases (2) increases (3) remains the sameJan 2003- 41 Which phase change represents a decrease in entropy?(1) solid to liquid (3) liquid to gas
(2) gas to liquid (4) solid to gas Aug 2002-39 Which sample has the lowest entropy?
(1) 1 mole of KNO3(l) (3) 1 mole of H2O(l)
(2) 1 mole of KNO3(s) (4) 1 mole of H2O(g) June 2002-44 Which process is accompanied by a decrease in entropy? (1) boiling of water
(2) condensing of water vapor
(3) subliming of iodine
(4) melting of ice | 
